Description
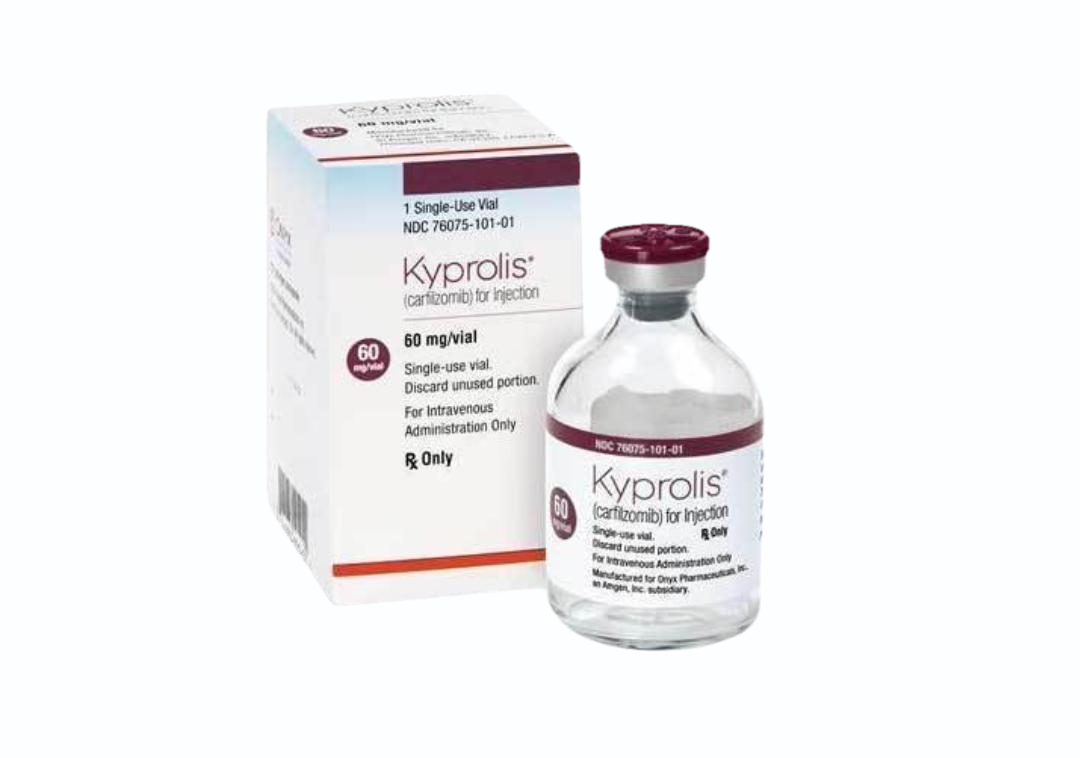
Carfilzomib Injection
The FDA approved carfilzomib in July 2012, for use in people with multiple myeloma who have received at least two prior therapies, including treatment with bortezomib and an immunomodulatory therapy (such as lenalidomide) and have demonstrated disease progression on or within 60 days of completion of the last therapy.

List Of Generic Brands For Carfilzomib Injection In India
If you are looking generic brands for Carfilzomib Injection in India. Please contact us at +91 9953466646 or drop us an email at sales@aarkpharma.com. Our representatives will be happy to assist you and answer any questions you may have Carfilzomib Injection Price is available at Aark Pharmaceuticals.